Contents
- 1 Q1. Which one of the following polymers is widely used for making bullet proof material? (1995)
- 2 Q2. Which one of the following pairs of materials serves as electrodes in chargeable batteries commonly used in devices such as torchlights, electric shavers etc. (1995)
- 3 Q3. Consider the following chemicals. (2006)
- 4 Q4. Which one of the following is another name of RDX? (2007)
- 5 Q5. What is the pH level of blood of a normal person? (2008)
- 6 Q6. Which one of the following pairs of metals constantly the lightest metal and the heaviest meta respectively?
- 7 Q7. With reference to the usefulness of the by-products of sugar industry, which of the following statements is/are correct? (2013)
- 8 Q8. Which of the following is/are the example/ examples of chemical change? (2014)
- 9 Q9. An aqueous solution of copper sulphate is acidic in nature because the salt undergoes (2001)
- 10 Q10. A radioactive substance has a half-life of four months. Three fourth of the substance would decay in (2001)
- 11 Q11. Assertion (A) A chemical reaction becomes faster at higher temperature. (2001)Reason (R) At higher temperature, molecular motion becomes more rapid.
- 12 Q12. Match List I (oxidation number) with List II (the element) and select the correct answer using the codes given below the lists. (2002)
- 13 Q13. With reference to ionic compounds, consider the following statements. 6001.
- 14 Q14. Which one of the following statements is correct? (2003)
- 15 In case you still have your doubts, contact us on 9811333901.
Q1. Which one of the following polymers is widely used for making bullet proof material? (1995)
(a) Polyvinyl chloride
(b) Polyamides
(c) Polyethylene
(d) Polycarbonates
Ans. (d)
Exp. Polymers widely used for making bullet proof material are polycarbonates which gives resistance to a bullet from penetrating. Polycarbonate polymer contains carbonate group
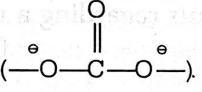
It is a durable material, have high temperature resistance, have transparency to visible light. Due to this reason, it is used for making bullet proof material, eye lenses etc.
Q2. Which one of the following pairs of materials serves as electrodes in chargeable batteries commonly used in devices such as torchlights, electric shavers etc. (1995)
(a) Nickel and cadmium
(b) Zinc and carbon
(c) Lead peroxide and lead
(d) Iron and cadmium
Ans. (a)
Exp. Statements (a) and (b) are correct.
Liquid sodium is used as a heat transfer fluid in some fast reactors, due to its high thermal conductivity and low neutron absorption. Moreover, the high boiling point allows the reactor to operate as coolant at ambient pressure.
Toothpastes are derived from a variety of components, one of the main component is abrasives. Abrasives constitute at least 50% of a typical toothpaste. These insoluble particles helps to remove plaque from the teeth. Representative abrasives include particles of aluminum hydroxide [Al(OH)], calcium carbonate (CaCO3) etc.
Bordeaux mixture is a mixture of copper (II) sulphate (CuSO4) and slaked lime [Ca(OH)2] used as a fungicide. An amalgam is a substance formed by the combination of mercury with another metal. In dentistry, amalgam is an alloy of mercury with various metals used for dental fillings. It commonly consists of mercury (50%), silver (~22-32%), tin (~14%), copper (~8%), and other trace metals.
Q3. Consider the following chemicals. (2006)
- Benzene
- Carbon tetrachloride
- Sodium carbonate
- Trichloroethylene
Which of the above is/are used as dry-cleaning chemical?
(a) Only 1
(b) Only 2
(c) 1, 2 and 4 only
(d) 1, 2, 3 and 4
Ans. (c)
Exp. In the given chemicals (1), (2) and (4) are correct. Benzene was historically used as a significant component in many consumer products such as liquid wrench, several paint strippers, rubber cements, spot removers and other hydrocarbon-containing products. Carbon tetrachloride has also been used as a dry-cleaning agent and fire extinguisher; in making nylons; as a solvent for rubber cement, soaps, insecticides etc. Trichloroethylene has also been used as a dry-cleaning solvent. But sodium carbonate cannot be used as dry-cleaning agent.
Q4. Which one of the following is another name of RDX? (2007)
(a) Cyanohydrin
(b) Dextran (c) Cyclohexane
(d) Cyclonite
Ans. (d)
Exp. Among the following, cyclonite is other name of RDX, hexogen and T4. RDX stands for Research and Development Explosive.
Chemical name of RDX is cyclotrimethylene trinitramine. It is also called plastic explosive. It is known as cyclonite in USA, hexogen in Germany and T4, in Italy.
Q5. What is the pH level of blood of a normal person? (2008)
(a) 4.5-4.6 (b) 6.45-6.55
(c) 7.35-7.45 (d) 8.25-8.35
Ans. (c)
Exp. Among the following, 7.35-7.45 is the pH level of blood of a normal person. pH stands for potential of hydrogen. The acidities and basicity of compounds are denoted by the pH value of their aqueous solutions. This is just a number (from 0 to 14) without any units, any solution with pH between 0 and 6.99 is acidic, while any solution with pH between 7.01 and 14 is basic. A solution
with a pH of 7 is neutral. (pH of water = 7)
Q6. Which one of the following pairs of metals constantly the lightest metal and the heaviest meta respectively?
(a) Lithium and mercury
(b) Lithium and osmium
(c) Aluminum and osmium
(d) Aluminum and mercury
Ans. (b)
Exp. Among the following, lithium is the lightest and osmium the heaviest metal. The atomic number, atomic weight and density of above-mentioned metal are as follows
| S.NO. | ELEMENTS | ATOMIC NUMBER | ATOMIC WEIGHT (amu) | DENSITY (G/CC) |
| 1 | LITHIUM | 3 | 6.941 | 0.534 |
| 2 | MERCURY | 80 | 200.592 | 13.534 |
| 3 | OSMIUM | 76 | 190.233 | 22.59 |
| 4 | ALUMINIUM | 13 | 26.982 | 2.70 |
Hence, it is clear that lithium (Li) is the lightest metal because its density is low, and osmium is the heaviest metal because its density is high.
Q7. With reference to the usefulness of the by-products of sugar industry, which of the following statements is/are correct? (2013)
- Bagasse can be used as biomass fuel for the generation of energy.
- Molasses can be used as one of the feedstocks for the production of synthetic chemical fertilisers.
- Molasses can be used for the production of ethanol.
Select the correct answer using the codes given below.
(a) Only 1
(c) 1 and 3
(b) 2 and 3
(d) All of these
Ans. (c)
Exp. Statements (1) and (3) are correct as Bagasse is the fibrous material, which is left over after juice is extracted from sugarcane. It is a good source of energy and usually produces enough electricity to power all of the mill’s operations.
Ethanol is produced by the fermentation of sugarcane and molasses. It is emerging as a leading additive to petrol-based fuels in the transportation sector.
Q8. Which of the following is/are the example/ examples of chemical change? (2014)
- Crystallization of sodium chloride
- Melting of ice
- Souring of milk
Select the correct answer using the codes given below.
(a) 1 and 2 (b) Only 3
(c) All of these (d) None of these
Ans. (b)
Exp. Souring of milk is a chemical change as bacteria activates the formation of lactic acid which turns milk sour. Crystallization of sodium chloride is a physical change, and no chemical transformation takes place. Melting of ice is also physical change. It changes into other physical form, i.e. liquid
Q9. An aqueous solution of copper sulphate is acidic in nature because the salt undergoes (2001)
(a) dialysis
(c) hydrolysis
(b) electrolysis
(d) photolysis
Ans. (c)
Exp. An aqueous solution of copper sulphate is acidic in nature because the salt undergoes hydrolysis. When copper sulphate (CuSO4) comes in contact with water (aqua, H₂O), the molecules of CuSO, break down and form a new compound with water which are copper hydroxide
[Cu(OH₂)] and sulphuric acid (H₂SO₄)
Q10. A radioactive substance has a half-life of four months. Three fourth of the substance would decay in (2001)
(a) 3 months (b) 4 months
(c) 8 months (d) 12 months
Ans. (c)
Exp. Three fourth of the substance would decay in 8 months. Half-life is the time period during which half of the substance decays. After 4 months substance remains = 1/2. After 8 months substrative remains 1/4. So, 3/4 of the substance would decay in 8 months.
Q11. Assertion (A) A chemical reaction becomes faster at higher temperature. (2001)
Reason (R) At higher temperature, molecular motion becomes more rapid.
Codes (a) Both A and R are true, and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct
explanation of A (c) A is true, but R is false
(d) A is false but R is true
Ans. (a)
Exp. Both A and R are true, and R is the correct explanation of A. Chemical reaction becomes faster at high temperature because at high temperature, molecules have high kinetic energy (energy associated with movement) and molecular
motion is higher than at normal temperature. Also due to high molecular motion chances of collision and hence chances of combination of molecules are also high.
Q12. Match List I (oxidation number) with List II (the element) and select the correct answer using the codes given below the lists. (2002)
| List I (oxidation number) | List II (the element) |
| A. 2 | 1. Oxidation number of Mn in MnO2 |
| B. 3 | 2. Oxidation number of S in H2S2O |
| C. 4 | 3. Oxidation number of Ca in CaO |
| D. 6 | 4. Oxidation number of Al in NaAlH4 |
Codes
A B C D A B C D
(a) 3 4 1 2 (b) 4 3 1 2
(c) 3 4 2 1 (d) 4 3 2 1
Ans. (a)
Exp. The correct matching is A→(3), В(4), C(1), D→(2)
Oxidation number of Mn in MnO2 x+(-2)x2=0 x-4=0
x = +4
Oxidation number of S in H2 S2 07 2×1+2xx+7x(-2)=0 2x-12-0
x = +6
Oxidation number of Ca in CaO x-2=0 x=+2
Oxidation number of Al in NaAlH 1+x+4x(-1)=0 x-3=0 x=+3
Q13. With reference to ionic compounds, consider the following statements. 6001.
- Ionic compounds are insoluble in alcohol. (2003).
- Ionic compounds in the solid state are good conductor of electricity.
Which of these statements is/are correct?
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Ans. (a)
Exp. Only statement (1) is correct. Ionic compounds (like table salt, NaCl) are readily soluble in water but insoluble in organic solvent like ether, alcohol, acetone. Ionic compounds in solution dissociate to form charged particles called ions (Na and Cl) and thus are good conductor of electricity but in solid state they are bad conductor because there are no free ions to carry charge.
Q14. Which one of the following statements is correct? (2003)
(a) Liquid sodium is employed as a coolant in nuclear reactors
(b) Calcium carbonate is an ingredient of toothpaste (c) Bordeaux mixture consists of sodium sulphate and lime
(d) Zinc amalgams are used
Ans. (a) and (b)
When coke reacts with oxide metals like cupric oxide (CuO), oxygen leave metal and form oxide with carbon (from coke). Thus metal (say copper) gets separated. Hydrogenation is basically adding hydrogen to a compound. When hydrogen gas is added to vegetable oil, the structure and properties of its molecules change. The new product formed remains solid at room temperature and is called edible fat or vanaspati ghee. The catalyst (a substaned which speeds up the rate of chemical reaction without taking part in it) used in this process is nickel (Ni). Vulcanisation process occur when sulphur is added to natural rubber in order to make it hard and durable. Natural rubber is soft, sticky and fragile. Hence it has limited applications. While the vulcanized rubber being stronger, harder more abrasion resistant and non-sticky find various application like tires, shoe soles, hoses, conveyor belts, gaming balls etc.
In case you still have your doubts, contact us on 9811333901.
For UPSC Prelims Resources, Click here
For Daily Updates and Study Material:
Join our Telegram Channel – Edukemy for IAS
- 1. Learn through Videos – here
- 2. Be Exam Ready by Practicing Daily MCQs – here
- 3. Daily Newsletter – Get all your Current Affairs Covered – here
- 4. Mains Answer Writing Practice – here

