Prepare for the UPSC Prelims with comprehensive topic-wise questions in Physics, focusing on the theme of Heat and Temperature. This specialized resource offers a curated selection of questions meticulously crafted to enhance your understanding of key concepts in this subject area. Dive deep into the nuances of heat transfer, thermal equilibrium, temperature scales, and more, as you sharpen your analytical skills and build a solid foundation in Physics. With clear explanations and a structured approach, this resource enables efficient and effective preparation for the UPSC Prelims exam. Whether you’re revisiting core principles or delving into advanced topics, these questions provide valuable practice to boost your confidence and performance on exam day. Elevate your preparation and maximize your success with this dedicated collection of UPSC Prelims Topic Wise Questions in Physics – Heat and Temperature.
Contents
- 1 Q1. The variations in temperatures from 0°C to 100°C with respect to time of two liquids P, Q are shown in the graph given below. (1995)
- 2 Q2. Strips of two metals A and B are firmly joined together as shown in the figure. (1999)
- 3 Q3. Assertion (A) A piece of copper and a piece of glass are heated to the same temperature. When touched, thereafter, the copper piece appears hotter than the glass piece. Reason (R) The density of copper is more than that of glass. (2001)
- 4 Q4. Assertion (A) The boiling point of water decreases a the altitude increases. Reason (R) The atmospheric pressure increases with altitude. (2001)
- 5 Q5. When water is heated from 0°C to 10°C. Its volume (2001)
- 6 Q6. A hollow sphere of radius R, a hollow cube of side R and a thin circular plate of radius R, made up of the same material, are all heated to 20°C above room temperature. When left to cool in the room, which of them will reach the room temperature first? (2002)
- 7 Q7. Consider the following statements. (2003)
- 8 Q8. What is the principle by which a cooling system (radiator) in a motor car works? (2010)
- 9 In case you still have your doubts, contact us on 9811333901.
Q1. The variations in temperatures from 0°C to 100°C with respect to time of two liquids P, Q are shown in the graph given below. (1995)
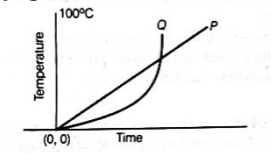
Which one of the following statements is correct?
(a) During heating, liquid P remain hotter than liquid e throughout
(b) At no point of time during heating did the two liquids have the same temperature
(c) Pattained the temperature of 100°C faster than Q
(d) Qattained the temperature of 100°C faster than P
Ans. (d)
According to the graph (temperature versus time), statement (d) is accurate. Q reaches the temperature of 100°C more rapidly than P does. This is evident from the graph, where the temperature of Q rises quickly, while the temperature of P increases at a consistent rate.
Q2. Strips of two metals A and B are firmly joined together as shown in the figure. (1999)

On heating, A expands more than B does. If this joined strip is heated, then it will appear as

Ans. (b)
The connected metal strips A and B, upon heating, will exhibit the configuration depicted in the figure provided in option (b). This occurs because when a bimetallic strip comprising two distinct metals is heated, the metal with greater expansion will be positioned on top, while the metal with lesser expansion will remain at the bottom. As a result, the strip curves downward.
Q3. Assertion (A) A piece of copper and a piece of glass are heated to the same temperature. When touched, thereafter, the copper piece appears hotter than the glass piece. Reason (R) The density of copper is more than that of glass. (2001)
Codes
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Ans. (b)
Both statements A and R are true, but statement R does not provide the correct explanation for statement A.
Statement A: When a piece of copper and a piece of glass are heated to the same temperature and then touched, the copper piece feels hotter than the glass piece.
Statement R: Copper is a good conductor of heat and electricity, while glass is not. Therefore, copper readily transfers heat to our body compared to glass.
Explanation: Statement A correctly identifies the observation that copper feels hotter than glass when both are heated to the same temperature. Statement R correctly identifies that copper is a better conductor of heat compared to glass. However, it fails to explain why copper feels hotter to the touch. The explanation provided in statement R only focuses on the conductivity of copper and glass, but it doesn’t address the specific mechanism by which copper’s higher conductivity results in the sensation of greater heat. Therefore, statement R does not provide a sufficient explanation for statement A.
Q4. Assertion (A) The boiling point of water decreases a the altitude increases. Reason (R) The atmospheric pressure increases with altitude. (2001)
Codes
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Ans. (c)
Statement A is true, but statement R is false.
Statement A correctly defines the boiling point of water and explains that it increases with an increase in pressure and vice versa. However, statement R incorrectly states that as altitude increases, air becomes less dense, leading to lower atmospheric pressure. In reality, as altitude increases, air density decreases, but it doesn’t necessarily lead to lower atmospheric pressure. The decrease in atmospheric pressure with altitude is primarily due to the decreasing weight of the air column above. Therefore, while the conclusion about the boiling point of water decreasing with altitude is correct, the explanation provided in statement R is inaccurate.
Q5. When water is heated from 0°C to 10°C. Its volume (2001)
(a) increases
(b) decreases
(c) does not change
(d) first decreases and then increases
Ans. (d)
When water is heated from 0°C to 10°C, its volume exhibits an anomalous behavior. Typically, substances expand when heated, but water deviates from this norm. This unusual behavior is referred to as the anomalous behavior of water. Specifically, as water is heated from 0°C to 4°C, its volume decreases (density increases), contrary to the usual trend. However, beyond 4°C, the volume of water starts to increase again (density decreases) with further heating. This peculiar pattern culminates in the maximum density of water occurring at 4°C.
Q6. A hollow sphere of radius R, a hollow cube of side R and a thin circular plate of radius R, made up of the same material, are all heated to 20°C above room temperature. When left to cool in the room, which of them will reach the room temperature first? (2002)
(a) Circular plate
(b) Cube
(c) Sphere
(d) All of them will reach the room temperature at the same time
Ans. (c)
When a hollow sphere, a hollow cube, and a thin circular plate are all heated to 20°C above room temperature and then left to cool in the room, the hollow sphere will reach room temperature first. This is because the sphere holds the maximum surface area exposed at 20°C above room temperature to heat up.
This can be explained based on the given conditions in the question. The surface area of the hollow sphere (inner and outer side) is calculated as As = 8πR^2, which is equal to 25.12R^2. The surface area of the hollow cube (inner and outer side) is calculated as Ac = 12R^2. The surface area of the circular plate is calculated as Ap = 2πR^2, which is equal to 6.28R^2.
It is evident that As > Ap > Ac. Since greater surface area results in more heat loss, the rate of heat loss for the sphere is maximum, followed by the plate, and then the cube. Therefore, the hollow sphere will cool down to room temperature faster than the cube and the plate.
Q7. Consider the following statements. (2003)
1. Steam at 100°C and boiling water at 100°C contain same amount of heat.
2. Latent heat of fusion of ice is equal to the latent heat of vaporisation of water.
3. In an air-conditioner, heat is extracted from the room air at the evaporator coils and is rejected out at the condenser coils.
Which of these statements is/are correct?
(a) 1 and 2
(b) 2 and 3
(c) Only 2
(d) Only 3
Ans. (d)
Statement 3 is accurate. In an air conditioning system, the evaporator coil functions to extract heat from the room air, which is then expelled by the condenser coil. The evaporator coil is responsible for absorbing heat from the indoor air, collaborating with the condenser coil to facilitate the heat exchange process that generates cool air.
However, statements 1 and 2 are incorrect. While it is true that steam at 100°C possesses more heat energy than boiling water at the same temperature, this is not due to specific latent heat of vaporization but rather the sensible heat of the steam. Latent heat of vaporization refers to the amount of heat required to convert a unit mass of a substance from liquid to vapor phase at constant temperature, not the total heat energy present in the steam.
Additionally, the statement about the latent heat of fusion of ice being 80 cal/g and the latent heat of vaporization of water being 536 cal/g is accurate. Latent heat represents the amount of heat absorbed or released per unit mass during a phase change without any change in temperature. The latent heat of fusion pertains to the solid-liquid phase change, while the latent heat of vaporization pertains to the liquid-gas phase change.
Q8. What is the principle by which a cooling system (radiator) in a motor car works? (2010)
(a) Conduction only
(b) Convection
(c) Radiation only
(d) Both conduction and radiation
Ans. (b)
A cooling system, such as a radiator in a motor car, operates based on the principle of forced convection. In this process, the radiator transfers the heat absorbed by the coolant into the atmosphere. Forced convection involves the active movement of heated material, facilitated by a blower or pump, to transfer heat from one location to another.
In case you still have your doubts, contact us on 9811333901.
For UPSC Prelims Resources, Click here
For Daily Updates and Study Material:
Join our Telegram Channel – Edukemy for IAS
- 1. Learn through Videos – here
- 2. Be Exam Ready by Practicing Daily MCQs – here
- 3. Daily Newsletter – Get all your Current Affairs Covered – here
- 4. Mains Answer Writing Practice – here