
The Indian pharmaceutical industry stands at a pivotal juncture, poised for a transformative shift from a traditional emphasis on volume to a more nuanced focus on value. Historically recognized for its substantial production capacity and generic drug manufacturing capabilities, the industry is now confronted with the imperative of attaining ‘value’ leadership to secure a formidable position in the global market. This evolution is not merely about increasing output but entails a comprehensive reorientation towards innovation, research and development, and the delivery of high-quality, differentiated products and services. The transition from quantity-driven growth to value-driven prominence is essential for the Indian pharma sector to navigate the increasingly competitive and dynamic international landscape, fostering sustainability and long-term success. This paradigm shift demands strategic foresight, investment in research and development, and an unwavering commitment to global standards of quality and efficacy, signaling the industry’s readiness to redefine its role in the world pharmaceutical market.
Answer
The Indian pharmaceutical industry is the third largest in the world by volume and the 14th largest in terms of value. Despite its manufacturing prowess, the industry has significant untapped innovation potential, which accounts for the majority of sector value globally.
Volume Leadership of the Indian Pharmaceutical Industry:
- It is one of the largest and most vibrant in the world.
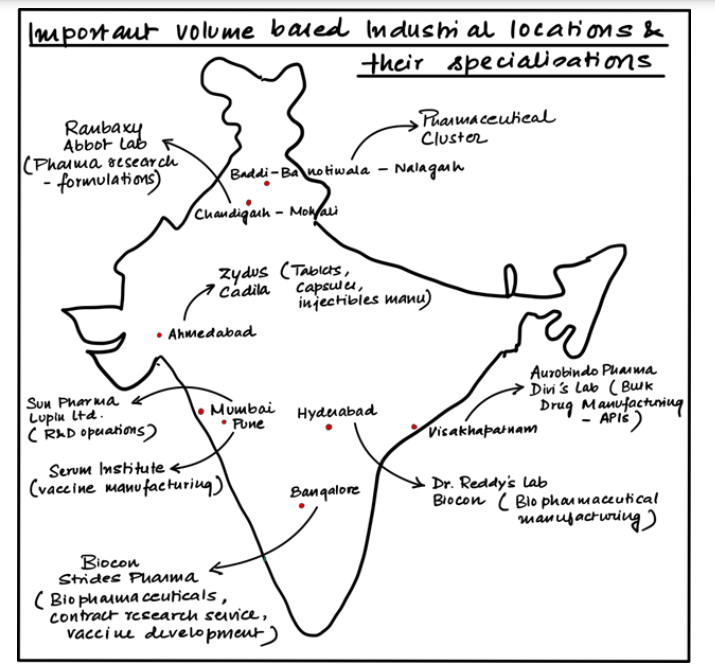

- Constitutes 3.5% of global drug and medical exports
- Largest supplier of generic medicines- 20% of global supply by volume.
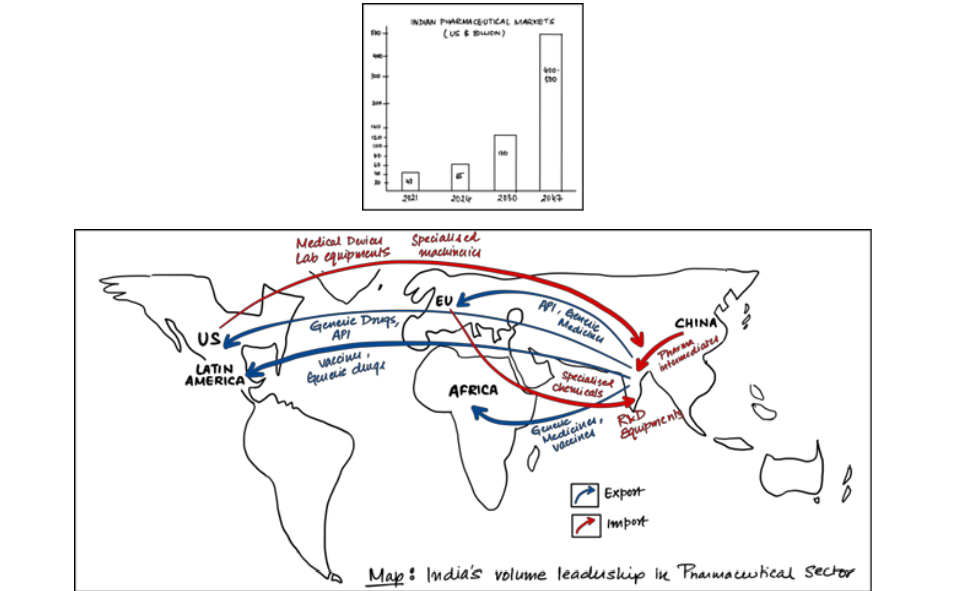
- Exported vaccines to 150+ countries during Covid 19
- Economic survey 2022-23 projects the Indian pharmaceutical industry to touch 400- 500 billion USD by 2047.
Moving from Volume to Value Leadership:
Problems that inhibit its move:
- Substandard Research Quality: Drugs have been launched without any rigorous pharmaceutical studies and meaningful clinical trials. Eg- export of contaminated cough syrup to Gambia.
- Research and development (R&D) investment Disparity: Indian Pharma companies allocate
around 8% of their revenues to R&D, whereas global counterparts typically invest 20-25%. - Multiple Regulatory Agencies: The current framework involves numerous regulatory bodies,
causing significant delays in approvals for pharmaceutical companies. - Current Indian Regulatory Standards are not aligned with global benchmarks like “International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use” (ICH) “Pharmaceutical Inspection Cooperation Scheme”(PIC)
- Neglect of treatment of Rare Diseases: Currently, very few pharmaceutical companies are
manufacturing drugs for rare diseases.
Hence, to move from Volume to Value Leadership, the need of the hour is:
- Increase investment in R&D. For example government has unveiled ₹5,000 crore
through the Promotion of Research & Innovation Scheme that aims to establish Centers of Excellence and stimulate private sector research. - Promotion of Ethical Research: Encouraging computer simulations and test tube studies that
reduces reliance on animal testing. - Aligning Indian regulatory standards with global benchmarks in research. For example- The
New Drugs, Medical Devices, and Cosmetics Bill, 2022 reflects India’s commitment to quality by emphasizing safety, effectiveness, and international compliance. - Strong regulatory framework that prioritizes patient-centricity. For example- the amendment to New Drugs and Clinical Trial Rules reflects India’s commitment to ethical research and innovation and prioritizes patient safety.
- Indian Pharma Industries can exploit the Rare Disease Market Segment. For example- Rashtriya Arogya Nidhi scheme aims at providing Financial support of up to Rs. 20 lakhs for rare diseases.
- Expanding the ambit of Pharmaceutical products to Alternative Medicines such as Ayurveda,
Yunani (AYUSH). For example- AYUSH Oushadhi Gunvatta Evam Uttpadan Samvardhan Yojana (AOGUSY) to promote and project quality, acceptability and visibility of AYUSH products for
enhancing people’s confidence in their use for health care and for improving trade - Streamlining Regulatory Processes to accelerate the transition from lab to market
- Striking the right balance between access, affordability, and innovation that is essential for
sustainable growth.
The Indian pharmaceutical sector is knowledge-driven and crucial to the country’s strategic goals. Transitioning from volume to value leadership requires a holistic approach encompassing quality, innovation, compliance, and strategic partnerships.
In case you still have your doubts, contact us on 9811333901.
For UPSC Prelims Resources, Click here
For Daily Updates and Study Material:
Join our Telegram Channel – Edukemy for IAS
- 1. Learn through Videos – here
- 2. Be Exam Ready by Practicing Daily MCQs – here
- 3. Daily Newsletter – Get all your Current Affairs Covered – here
- 4. Mains Answer Writing Practice – here

