- The amount of nutrients, such as carbon, nitrogen, phosphorus, calcium, etc., present in the soil at any given time, is referred to as the standing state. The movement of nutrient elements through the various components of an ecosystem is called nutrient cycling. Another name of nutrient cycling is biogeochemical cycles (bio: living organism, geo: rocks, air, water). Nutrient cycles are of two types: (a) gaseous and (b) sedimentary.
- The reservoir for gaseous type of nutrient cycle (e.g., nitrogen, carbon cycle) exists in the atmosphere and for the sedimentary cycle (e.g., sulphur and phosphorus cycle), the reservoir is located in Earth’s crust.
- Environmental factors, e.g., soil, moisture, pH, temperature, etc., regulate the rate of release of nutrients into the atmosphere. The function of the reservoir is to meet with the deficit which occurs due to imbalance in the rate of influx and efflux.
Carbon Cycle
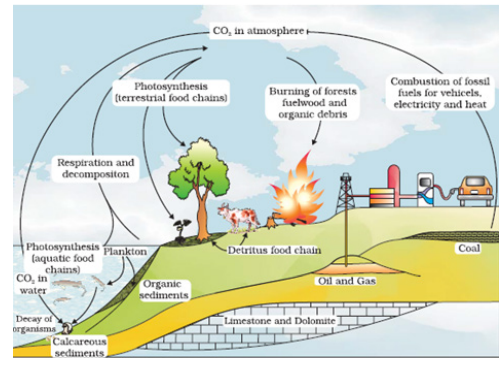
- Carbon constitutes 49 per cent of dry weight of organisms and is next only to water. If we look at the total quantity of global carbon, we find that 71 per cent carbon is found dissolved in oceans. This oceanic reservoir regulates the amount of carbon dioxide in the atmosphere.
- Fossil fuels also represent a reservoir of carbon. Carbon cycling occurs through atmosphere, ocean and through living and dead organisms. According to one estimate 4 × 1013 kg of carbon is fixed annually in the biosphere through photosynthesis. A considerable amount of carbon returns to the atmosphere as CO2 through respiratory activities of the producers and consumers. Decomposers also contribute substantially to CO2 pool by their processing of waste materials and dead organic matter of land or oceans. Some amount of the fixed carbon is lost to sediments and removed from circulation.
- Burning of wood, forest fire and combustion of organic matter, fossil fuel, volcanic activity are additional sources for releasing CO2 in the atmosphere. Human activities have significantly influenced the carbon cycle. Rapid deforestation and massive burning of fossil fuel for energy and transport have significantly increased the rate of release of carbon dioxide into the atmosphere.
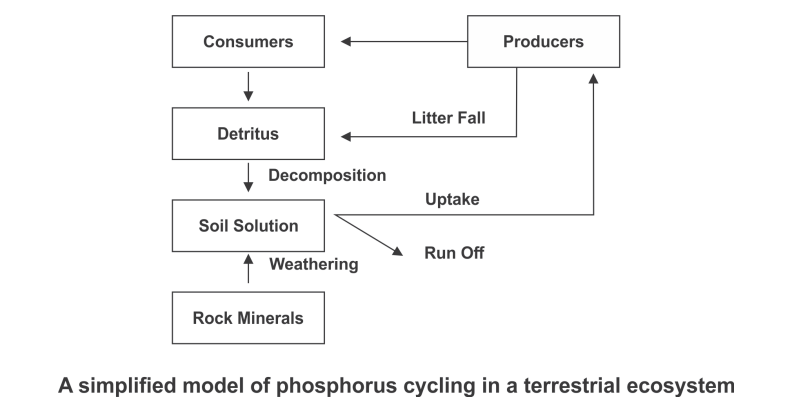
Phosphorus Cycle
- Phosphorus is a major constituent of biological membranes, nucleic acids and cellular energy transfer systems. Many animals also need large quantities of this element to make shells, bones and teeth. The natural reservoir of phosphorus is rock, which contains phosphorus in the form of phosphates. When rocks are weathered, minute amounts of these phosphates dissolve in soil solution and are absorbed by the roots of the plants. Herbivores and other animals obtain this element from plants. The waste products and the dead organisms are decomposed by phosphate-solubilising bacteria releasing phosphorus. Unlike carbon cycle, there is no respiratory release of phosphorus into atmosphere.
- The other two major and important differences between carbon and phosphorus cycle are firstly, atmospheric inputs of phosphorus through rainfall are much smaller than carbon inputs, and, secondly, gaseous exchanges of phosphorus between organism and environment are negligible.
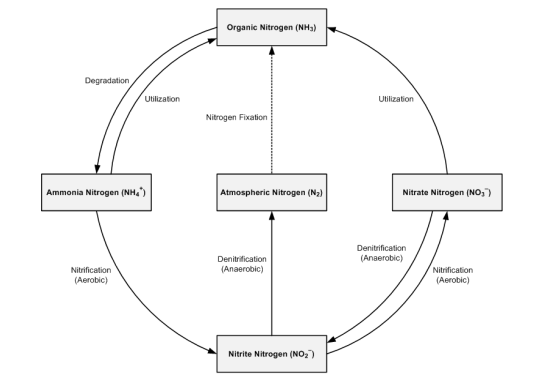
Nitrogen cycle
- Nitrogen is an essential component of protein and required by all living organisms including human beings. Our atmosphere contains nearly 79% of nitrogen but it cannot be used directly by the majority of living organisms.
- Broadly like carbon-dioxide, nitrogen also cycles from gaseous phase to solid phase then back to gaseous phase through the activity of a wide variety of organisms. Cycling of nitrogen is vitally important for all living organisms. There are five main processes which essential for nitrogen cycle are elaborated below.
(a) Nitrogen fixation: This process involves conversion of gaseous nitrogen into Ammonia, a form in which it can be used by plants. Atmospheric nitrogen can be fixed by the following three methods:-
- Atmospheric fixation: Lightening, combustion and volcanic activity help in the fixation of nitrogen.
- Industrial fixation: At high temperature (400oC) and high pressure (200 atm.), molecular nitrogen is broken into atomic nitrogen which then combines with hydrogen to form ammonia.
- Bacterial fixation: There are two types of bacteria-
- Symbiotic bacteria e.g. Rhizobium in the root nodules of leguminous plants.
- Free living or symbiotic e.g. Nostoc, Azobacter and Cyanobacteria can combine atmospheric or dissolved nitrogen with hydrogen to form ammonia.
(b) Nitrification: It is a process by which ammonia is converted into nitrates or nitrites by Nitrosomonas and Nitrococcus bacteria respectively. Another soil bacterium Nitrobacter can covert nitrate into nitrite.
(c) Assimilation: In this process nitrogen fixed by plants is converted into organic molecules such as proteins, DNA, RNA etc. These molecules make the plant and animal tissue.
(d) Ammonification: Living organisms produce nitrogenous waste products such as urea and uric acid. These waste products as well as dead remains of organisms are converted back into inorganic ammonia by the bacteria.This process is called ammonification. Ammonifying bacteria help in this process.
(e) Denitrification: Conversion of nitrates back into gaseous nitrogen is called denitrification. Denitrifying bacteria live deep in soil near the water table as they like to live in oxygen free medium. Denitrification is reverse of nitrogen fixation.
Hydrological Cycle
- Water is a cyclic resource. It can be used and re-used. Water also undergoes a cycle from the ocean to land and land to ocean. The hydrological cycle describes the movement of water on, in, and above the earth. The hydrological cycle, is the circulation of water within the earth’s hydrosphere in different forms i.e. the liquid, solid and the gaseous phases. It also refers to the continuous exchange of water between the oceans, atmosphere, land surface and subsurface and the organisms. Following are the stages:
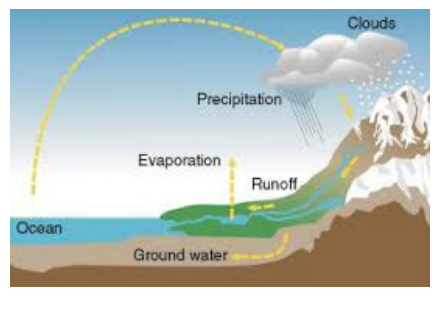
- Evaporation: It is one of the major processes in the cycle, is the transfer of water from the surface of the Earth to the atmosphere. By evaporation, water in the liquid state is transferred to the gaseous, or vapour, state. The main factors affecting evaporation are temperature, humidity, wind speed, and solar radiation. The principal source of water vapour is the oceans, but evaporation also occurs in soils, snow, and ice. Evaporation from snow and ice, the direct conversion from solid to vapour, is known as sublimation. Transpiration is the evaporation of water through minute pores, or stomata, in the leaves of plants. For practical purposes, transpiration and the evaporation from all water, soils, snow, ice, vegetation, and other surfaces are lumped together and called evapotranspiration, or total evaporation.
- Condensation: The transition process from the vapour state to the liquid state is called condensation. Condensation may take place as soon as the air contains more water vapour than it can receive from a free water surface through evaporation at the prevailing temperature. This condition occurs as the consequence of either cooling or the mixing of air masses of different temperatures. By condensation, water vapour in the atmosphere is released to form precipitation.
- Precipitation that falls to the Earth is distributed in four main ways: some is returned to the atmosphere by evaporation, some may be intercepted by vegetation and then evaporated from the surface of leaves, some percolates into the soil by infiltration, and the remainder flows directly as surface runoff into the sea. Some of the infiltrated precipitation may later percolate into streams as groundwater runoff.
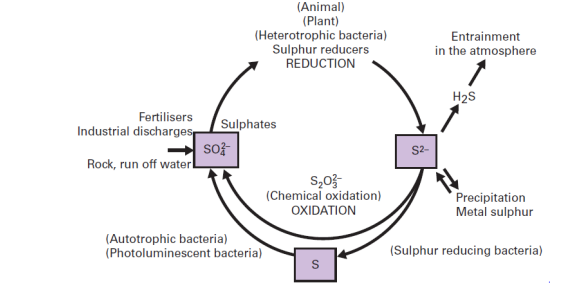
Sulphur Cycle
- The sulphur cycle is mostly sedimentary except for a short gaseous phase. The large sulphur reservoir is in the soil and sediments where it is locked in organic (coal, oil and peat) and inorganic deposits (pyrite rock and sulphur rock) in the form of sulphates, sulphides and organic sulphur. It is released by weathering of rocks, erosional runoff and decomposition by bacteria and fungi of organic matter and is carried to terrestrial and aquatic ecosystems in salt solution. Sulphur is found in gaseous forms like hydrogen sulphide and sulphur dioxide in small quantities in the atmosphere, which is thus a small reservoir. Sulphur enters the atmosphere from several sources like volcanic eruptions, combustion of fossil fuels, from surface of ocean and from gases released by decomposition. Hydrogen sulphide also gets oxidised into sulphur dioxide (SO2 ). Atmospheric SO2 is carried back to the earth after being dissolved in rainwater as weak sulphuric acid (H2 SO4 ). Whatever the source sulphur in the form of sulphates (SO-24) is taken up by plants and incorporated through a series of metabolic processes into sulphur bearing amino acids which are incorporated in the proteins of tissues of autotrophs. It then passes through the grazing food chain. Sulphur bound in living organism is carried back to the soil, to the bottom of ponds and lakes and seas through excretion and decomposition of dead organic material. Under aerobic conditions fungi like Aspergillus and Neurosporaand under anaerobic conditions the bacteria like Escherichia and Proteus are largely responsible for the decomposition of proteins.